Tools
Genetics/Models
To link synaptic and neural function to the molecular level, we take advantage of the versatile genetics toolbox available in Drosophila melanogaster, including genome-wide CRISPR-Cas9, transposon insertions, or RNAi. This allows us to implicate new molecular mechanisms in neural function through electrophysiology-based genetic screens. In a next step, the genes identified in Drosophila are tested in knock-out mice. We also study cultured Drosophila cells, as well as neuronal cultures derived from rodents or human iPSCs.
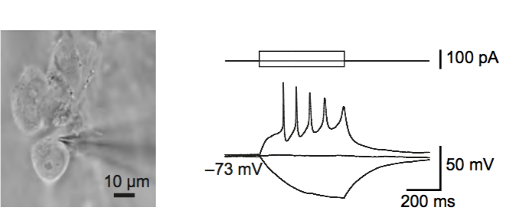
Electrophysiology
We use a variety of electrophysiology approaches to study synaptic transmission, intrinsic excitability, and ion channel function, ranging from two-electrode voltage clamp (TEVC) recordings in Drosophila to presynaptic whole-cell patch clamp recordings and membrane capacitance measurements in acute mouse brain slices.
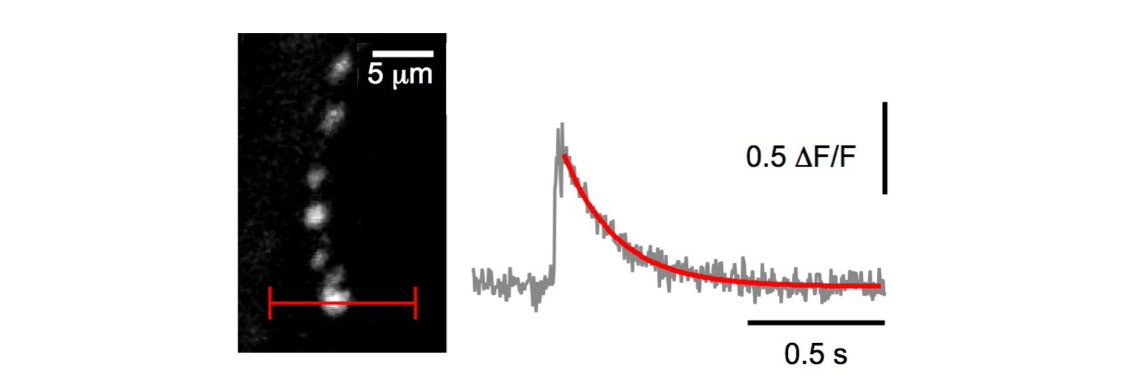
Functional imaging
We study presynaptic Ca++ dynamics after single AP stimulation at high temporal resolution using 2P Ca++ imaging, and started probing kinase activity using FRET sensors. Finally, we are in the process of validating a new optogenetic sensor for synaptic transmission.
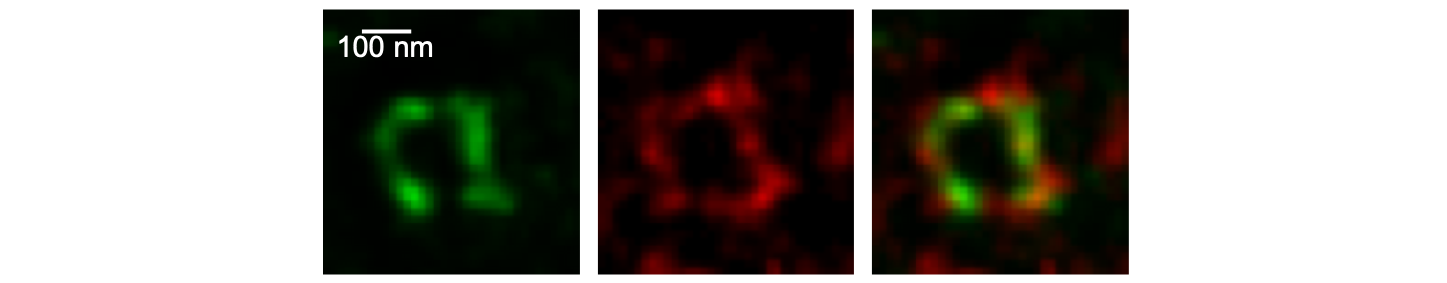
Microscopy
Besides studying synaptic structure and function with confocal and 2P microscopy, we employ STED imaging to investigate synaptic nano-architecture.
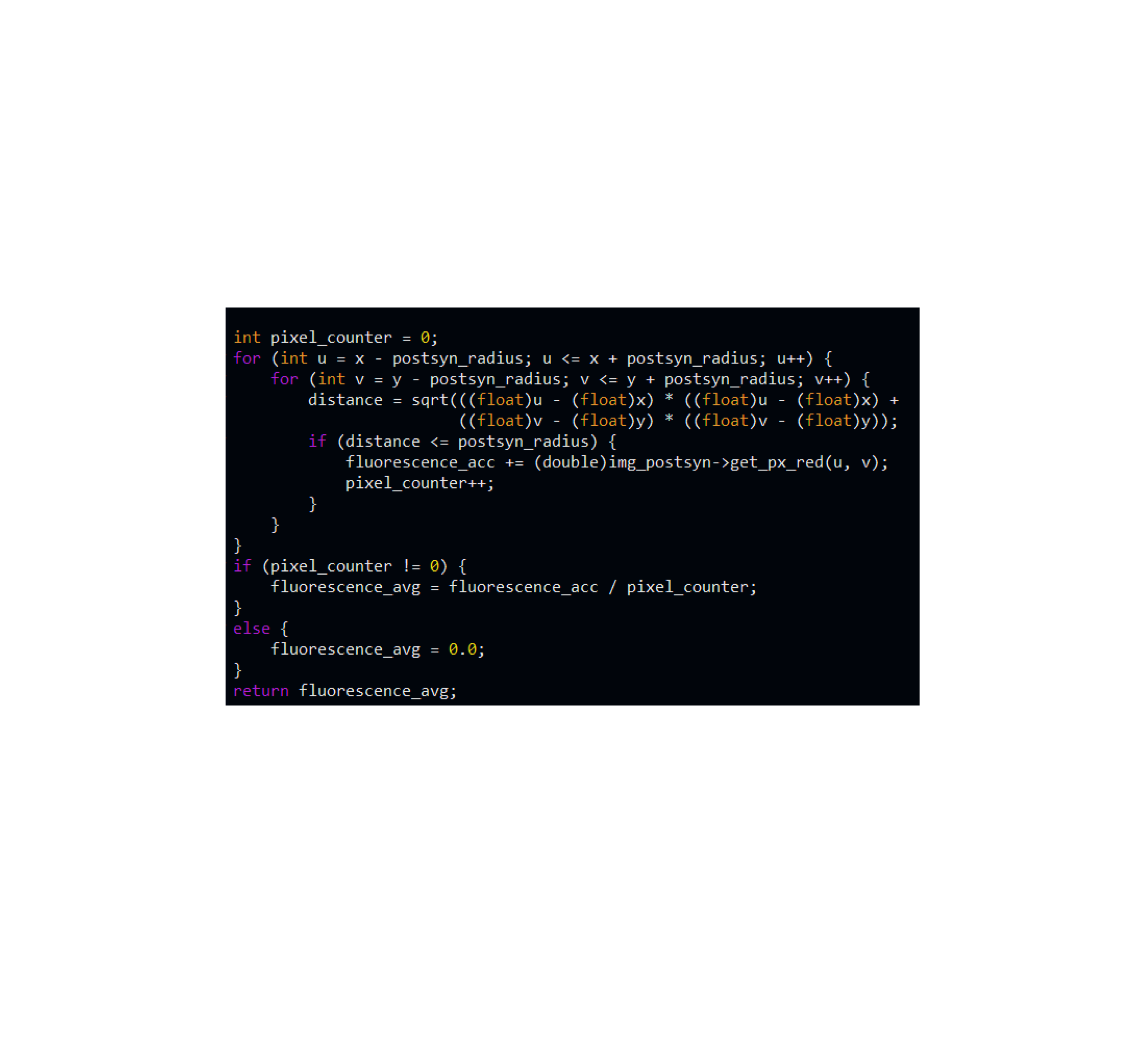
Quantitative analysis
We strongly believe in quantitative analysis and write code in IgorPro, Python, C++, ImageJ/Fiji, or R to analyze electrophysiology and imaging data.